Shigella campylobacter salmonella and e coli
Data from rapid diagnostic tests included in total infections for the first time
Embargoed Until: Thursday, April 20, 2017, 1:00 p.m. ET
Contact: Media Relations
(404) 639-3286
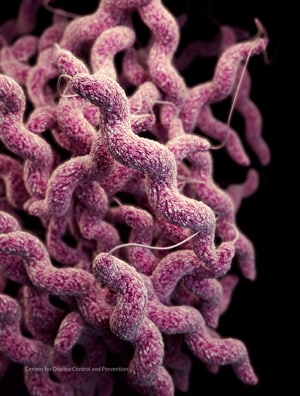
Campylobacter and Salmonella caused the most reported bacterial foodborne illnesses in 2016, according to preliminary data published today in CDC’s Morbidity and Mortality Weekly Report. CDC’s Foodborne Diseases Active Surveillance Network (FoodNet) report provides the most up-to-date information about foodborne illnesses in the United States.
FoodNet collects data on 15 percent of the U.S. population. FoodNet sites alone reported 24,029 foodborne infections, 5,512 hospitalizations, and 98 deaths in 2016. The numbers of reported illnesses by germ are: Campylobacter (8,547), Salmonella (8,172), Shigella (2,913), Shiga toxin-producing E. coli (1,845), Cryptosporidium (1,816), Yersinia (302), Vibrio (252), Listeria (127) and Cyclospora (55). This is the first time the report also includes in the total number of infections those foodborne bacterial infections diagnosed only by rapid diagnostic tests in FoodNet sites. Previously, the report counted foodborne bacterial infections confirmed only by traditional culture-based methods in the total numbers.
Salmonella Typhimurium infections, often linked to beef and poultry, decreased 18 percent in 2016 compared with the average for 2013-2015. The continuing decreases in Salmonella Typhimurium may be due to regulatory action to reduce Salmonella contamination in poultry and vaccination of chicken flocks by producers. Reported Yersinia, Cryptosporidium, and Shiga toxin-producing E.coli infections increased. These increases are likely due to newly available rapid tests that make infections easier to diagnose, rather than to a true increase in illness.
“This report provides important information about which foodborne germs are making people sick in the United States,” said Robert Tauxe, M.D., M.P.H, director of CDC’s Division of Foodborne, Waterborne, and Environmental Diseases. “It also points out changes in the ways clinicians are testing for foodborne illness and gaps in information as a result.”
Rapid tests speed treatment but miss important information
The new data reflect the increasing popularity of rapid tests known as culture-independent diagnostic tests, or CIDTs. These faster tests can have immediate benefits for treatment, but do not collect information needed to determine if an infection is antibiotic-resistant or if it is linked to an outbreak. Positive results on rapid tests can be followed up by culture-based tests to get detailed data, but often are not, according to the report.
“We need foodborne-illness trend data to monitor progress toward making our food supply safer,” Tauxe said. “It’s important that laboratories continue to do follow-up cultures on CIDT-positive patients so public health officials can get the information needed to protect people from foodborne illness.”
Foodborne illness remains a substantial public health concern in the United States. Previous analyses have indicated that the number of infections far exceeds those diagnosed; CIDTs might be making those infections more visible. However, the shift to CIDTs poses challenges to monitoring foodborne illness trends because changes in the number of new infections could reflect changes in testing practices rather than a true increase in infections. For this reason, comparisons of the 2016 data with data from previous years may not accurately reflect trends. Estimated infections this year and in years past are accurate, but cannot be directly compared because the total now includes results from diagnostic tests. FoodNet is developing new tools that will allow it to continue to track the needed progress toward reducing foodborne illness.
Improving food safety
FoodNet provides the information needed for effective food-safety policies and prevention efforts. CDC works closely with other federal, state, and local partners and with the food industry to improve food safety in the United States.
“We are making progress in detecting and responding more quickly to foodborne illness, but our priority remains preventing illnesses from happening in the first place,” said Susan Mayne, Ph.D., F.A.C.E., director of FDA’s Center for Food Safety and Applied Nutrition. “The final rules we are implementing under the FDA Food Safety Modernization Act focus on prevention, and we will continue to work closely with other government agencies at the local, state and federal levels, as well as our tribal and territorial partners, to support industry compliance with the new requirements.”
In 2016, USDA’s Food Safety and Inspection Service (FSIS) finalized new performance standards for reducing harmful bacteria in chicken parts and ground poultry. FSIS expects these actions could prevent as many as 50,000 illnesses each year caused by Salmonella and Campylobacter in chicken and turkey products.
“Our new performance standard for chicken parts is a perfect example of the type of proactive, prevention-based food policies that we’re focused on at FSIS – policies that are based on science, that are supported by strong data, and that will truly improve public health,” said Al Almanza, FSIS Administrator.
About FoodNet
FoodNet collects the information needed to track disease rates and determine trends in laboratory-confirmed illnesses caused by nine germs transmitted commonly by food: Campylobacter, Cryptosporidium, Cyclospora, Listeria, Salmonella, Shiga toxin-producing E. coli, Shigella, Vibrio, and Yersinia. Each year, FoodNet publishes a report that includes preliminary data compared with data from the previous three years. FoodNet has been monitoring illness trends since 1996.
Информация об исследовании
ПЦР (полимеразно- цепная реакция) - это метод, который позволяет найти в клиническом исследуемом материале небольшой участок генетической информации (ДНК) любого организма среди огромного количества других участков и многократно размножить его.
Биологический материал: кал.
Правила подготовки: кал следует сдавать до начала приема антибиотиков и химиотерапевтических препаратов (если это невозможно, то не ранее чем через 12 часов после отмены препарата).
Энтероинвазивные E.coli.
Кишечная палочка(E.coli) - возбудитель эшерихиозов, основная аэробная часть микрофлоры кишечника. Это граммотрицательные палочковые бактерии, принадлежащие к семейству Enterobacteriaceae. E.coli является обычным обитателем кишечника многих млекопитающих и приматов, к числу котрых принадлежит человек. Поэтому её называют кишечной палочкой. В организме человека кишечная палочка выполняет полезную роль, подавляя рост вредных бактерий и синтезируя некоторые витамины. Однако существуют разновидности бактерий E.coli способных вызывать у человека острые кишечные заболевания. В настоящее время выделяют более 150 типов патогенных (энтеровирулентных) палочек E.coli, объеденённых в 4 класса:
-Энтеропатогенные (ЭПЭК)
-Энтеротоксигенные (ЭТЭК)
-Энтероинвазивные (ЭНЭК)
-Энтерогемморагические (ЭГЭГ)
Энтероинвазивные кишечные палочки - возбудители поражений весьма напоминающих бактериальную дизентерию.Патогенез тоже носит черты явного сходства: подобно шигеллам энтероинвазивные кишечные палочки проникают и размножаются в клетках эпителия кишечника. Как и шигеллы они неподвижны и не способны ферментировать лактозу.
Поражения характеризуются выраженными болями в животе и профузной водянистой диареей с примесью крови. На инвазивность указывает большое количество полиморфных ядерных лейкоцитов в испражнениях. Путь передачи: фекально-оральный.
Источник инфекции:
-Больной человек и животные
-Бактериносители
-Фекально-загрязнённые продукты питания и вода
Одним из подтверждающих методов диагностики эшерихиоза является ПЦР.
Биологический материал: кал.
Правила подготовки: кал сдается до начала приема антибиотиков и химиотерапевтических препаратов (если это невозможно, то не ранее чем через 12 часов после отмены препарата).
Сальмонелла (Salmonella spp.)
Сальманеллёзы - острые кишечные инфекции животных и человека, вызываемые сальмонеллами. Salmonella spp - это подвижные, грамотрицательные палочки, принадлежащие к роду Salmonella, семейства Enterobacteriacea (энтеробактерии).
Источник инфекции:
-Больные животные
-Больной человек
-Бактериносители
Путь передачи: алиментарный - через инфицированные пищевые продукты, как правило животного происхождения (мясо, мясные продукты, молоко, яйца, особенно утиные и гусиные, студень), при вынужденном неправильном убое животных, нарушений правил хранения и приготовления продуктов (соприкосновение готовой и сырой продукции, недостаточная термическая обработка продуктов перед употреблением и т.д.).
Клиническая картина.
Инкубационный период колеблется от 2-6 часов до 2-3 суток. Клинические проявления сальмонеллезов от бессимптомного носительства возбудителя инфекции до тяжелых септических форм.
Правила подготовки: кал следует сдавать до начала приема антибиотиков (если это невозможно, то не ранее чем через 12 часов после отмены препарата).
Кампилобактерии (Campylobacter spp.)
Кампилобактериоз - это острое инфекционное зоонозное заболевание, характеризующееся синдромом общей интоксикации, поражением желудочно-кишечного тракта и возможностью генерализации патологического процесса.
Кампилобактерии (Campylobacter spp.) представители семейства Campylobacteriaceae - мелкие необразующие спор грамотрицательные палочки.
В настоящее время в состав семейства Campylobacteriaceae входит три рода: Campylobacter, Helicobacter, Arcobacter.
Эпидемиология.
Клиническая картина.
Инккубационный перид: 1-10 дней (чаще 2-5 дней).
Abstract
There are multiple etiologies responsible for infectious gastroenteritis causing acute diarrhea which are often under diagnosed. Also acute diarrhea is one of the major causes of morbidity and mortality among children less than 5 years of age.
In our study, fecal samples (n = 130) were collected from children (
Background
Global and national estimates clearly indicate that diarrheal disease is a major public health concern [1, 2]. According to the World Health Organization (WHO), diarrheal diseases are the second leading cause of death (
760,000 per year) in children
Methods
This study was conducted in a tertiary care teaching hospital in Bhubaneswar, Odisha (India). Fecal samples were collected from 130 children from June 2015 to April 2016. Stool samples were collected from children considering the inclusion and exclusion criteria. Inclusion criteria were children less than 5 years of age and 3 or more than 3 diarrheal episodes in a day. Children having malabsorption, immunocompromised individuals, and patients who have undergone immune suppressive therapy and prolonged steroid treatment were excluded from our study. Fecal samples were also collected from 48 non-diarrheal children less than 5 years of age, which acted as a control group. The study protocol was reviewed and approved by the Institutional Ethics Committee. Informed consent and patient datasheets were maintained for each participant.
From each of these participants, fecal samples were collected in a sterile vial. Samples were placed on ice and transported to lab within 4 h and processed immediately. Total fecal genomic DNA was extracted from stool using QIAamp Fast DNA stool Mini Kit (Qiagen, Germany) according to the manufacturer’s instruction. The quantity and purity of extracted DNA were measured using a Nanodrop (Epoch BioTek, USA).
Extracted DNA samples were stored at −20 °C until further processing. Bacterial genomic DNA was isolated from pure cultures of S. enterica subsp., serovar typhimurium, V. cholera, S. flexneri, and E. coli (from the repository) using blood and tissue genomic DNA isolation kit (Qiagen, Germany) as per the manufacturer’s protocol. These were used as positive controls for pathogen detection by polymerase chain reaction (PCR).
Fecal specimens were screened for the presence of Rotavirus and Adenovirus by using immunochromatographic test (Combi-Strip C-1004, Coris Bioconcept ltd- Belgium) in accordance with the manufacturer’s instructions. This is a rapid diagnostic test based on homogenous membrane system with colloidal gold particles. The test was carried out as described previously [22].
Fecal genomic DNA extracted from stool was used as a template in a series of PCR amplification reactions using primers specific for each pathogen (Table 1). Bacteria-specific genomic DNA isolated from pure cultures was used as a positive amplification control in PCR screening. For detecting protozoan parasites, positive DNA control for Cryptosporidium was obtained from Christian Medical College, Vellore (India), while standard Giardia DNA was obtained from the Institute of Parasitology, University of Zurich. PCR cycling conditions for different bacterial and protozoan parasites were as follows: Initial denaturation at 95 °C for 5 min, followed by 34 cycles of denaturation of 94 °C for 30 s, annealing at primer-specific temperature at 30–45 s and extension at 72 °C for 1 min, and final extension for 72 °C for 7 min. All PCR products were subjected to 1–1.5% agarose gel electrophoresis to confirm positive samples.
To examine associations between the presence or absence of diarrheal pathogen and risk factors within the different age groups (
Results
In the present study, overall result showed the highest detection rate for diarrheagenic E. coli (DEC) followed by Rotavirus and Shigella spp. in cases with symptoms of diarrhea (Table 2). Besides DEC (30.7%), the other pathogens with a decreasing order of detection rates were Rotavirus (26.15%), Shigella (23.84%), Adenovirus (4.61%), Cryptosporidium (3.07%), and Giardia (0.7%). Different strains of DEC such as EPEC (21.53%), STEC (10.76%), EAEC (6.90%), 0157 (4.61%), and EHEC (0.77%) were detected in the stool samples from cases. All these samples were negative for Salmonella spp. and Vibrio cholera. Surprisingly DEC, Shigella spp. and Adenovirus were also detected in 20.83, 4.61, and 2.08% of the healthy control subjects, respectively (Table 2). Representative gel image and immunochromatographic test positive strips are shown in Additional file 1.
Among the control subjects for which DEC was detected, the majority had infections with EPEC (10.41%) followed by STEC (6.25%), EAEC (2.08%), and O 157 (2.08%). No healthy subject was detected with Rotavirus, Giardia, Cryptosporidium spp., Salmonella spp., and Vibrio spp. The pathogen detection rates for cases and controls for each pathogen category are statistically analyzed which showed a significantly higher detection rate for Shigella spp (p = 0.0086) and Rotavirus (p = 0.0135), whereas the differences were not significant for rest of the detected pathogens.
Gender-wise distributions of diarrheal incidences for different etiological agents are shown in Table 3. The total detection rate for at least one infectious etiology among the cases with acute diarrhea was slightly lower in male patients (54.28%) than in female patients (60%). Only Rotavirus detection was significantly higher among male cases (p = 0.0003). Among all the cases with diarrhea, detection rate of DEC was slightly higher among males (31.42%) compared to females (30%). Cryptosporidium, Shigella, and Giardia were also detected with relatively higher rate in males compared to females. However, Adenovirus detection rate was moderately higher among females though the difference was not significant on statistical analysis.
Among cases with diarrhea, at least one infectious etiology was detected in 24 of 40 (60%) children living in rural settings in comparison to 50 of 90 (55.55%) children from urban settings. Adenovirus was more prevalent in rural children (p = 0.026) (Table 4). Cryptosporidium was also detected with higher rate among rural children when compared to urban ones (p = 0.02623). Though DEC was also detected predominantly in rural children, the difference however was not significant (p > 0.05). Among DEC, only STEC was found significantly higher (p = 0.014) in rural children (Additional file 2). In contrast, Rotavirus was more prevalent in urban children (p = 0.027). Shigella and Giardia detection rates were only slightly higher among the urban in comparison to rural children but not statistically significant (p > 0.05).
The data on age group ( 0.05). Of all etiologies, only Rotavirus infection was found significantly associated with children under Table 5 Age group-wise distribution of incidences of different etiological agents causing diarrhea in children in diarrheal group
Among the different DEC strains, EPEC was detected significantly higher in 2 years age group (p = 0.001) (Table 6), while no significant differences were observed on detection rates of STEC and EAEC when compared between the above two age groups. Moreover, E. coli O157 was detected with higher frequency among 1 year of age (data not shown).
The detection rates and their distribution among each individual pathogen type (only a single pathogen detection) comparing cases and controls are shown in the bar graphs as in Fig. 1. There was a significantly higher rate of detection only for Rotavirus mono-infection among cases with diarrhea in comparison to the controls (p = 005014). Also the overall rate of detection of a single infection only was significantly higher among cases in comparison to control subjects (p = 0.0268).
In this study, we observed many children infected with multiple pathogens, and the detection rates under different combinations of concurrent infections are presented in Table 7. Overall result showed simultaneous detection of two or more pathogens in 30% of cases included in this study. Of a total 44 cases with co-infections, 33 had double infections, 10 had triple infection, and only 1 had infection with more than three pathogens concurrently. Surprisingly, in case of control group of 48 children with no diarrhea, co-infections were detected in 3 (6.25%) children.
An overall analysis showed statistically significant differences between detection rates for combinations of two infections concurrently among cases vs controls (p = 0.004915). Co-infection of Rotavirus with Shigella was the most frequent combination, which was detected in 5.38% cases, followed by Rotavirus with EPEC (4.61%) and Shigella with STEC (3.84%). Co-infections with detection of two pathogens were detected in one healthy control child each with the following combinations, viz., EPEC with Shigella, EPEC with STEC, and EPEC with O 157.
Overall analysis also showed statistically significant differences between detection rates for combinations of three infections concurrently among cases vs controls (p = 0.03917). There were two cases each under the following combinations with triple infection: (1) Rotavirus and Shigella with STEC; (2) Shigella and STEC with EPEC. Other combinations of triple infections were detected only in one child in each combination of concurrent infections (Table 7).
Age group-wise distributions of mono-infections and multiple infections are shown in Fig. 2. Of the total 30 cases with detection of a single infection, there was a predominance under the younger age group ( Fig. 2
Overall data on disease severity (only in terms of numbers of liquid motions per day) compared between two groups, i.e., cases with detection of single infection vs cases with detection of more than one infections, are presented in Table 8. Two-sample independent t test analysis revealed significantly higher number of motions per day among cases with multiple infections compared to those with single infection based on both equal variance (p = 0.001015) and unequal variance (p = 0.006885). Other major associated symptoms were vomiting (46%) and fever (23%); when the symptoms were correlated with the test positivity for different etiologies, majority of cases positive for Shigella and Rotavirus had vomiting (29%) and fever (27%) (data not shown).
Discussion
Infectious diarrhea is a frequent problem in low-income countries which is a leading cause of death among children under 5 years of age [3]. Though many different types of agents are reported to be associated with infectious diarrhea, DEC has however been known to be the most commonly diagnosed etiology in India [30]. The overall result in our study showed that 56.92% of the children with diarrhea were diagnosed to be positive for at least one infectious etiology among the children with diarrhea. A study in the past showed E. coli (different strains) to be responsible for as much as 25% of all diarrheal diseases in developing countries [31]. In this study, we also found DEC to be the most common diarrheal agent in the study population.
In the diarrheal group, more than 1 infectious agent was diagnosed in 44 (33.84%) cases. In our study, we found Rotavirus+Shigella to be the most frequent co-infection combination, while a previous study from Leon Nicaragua reported EAEC along with EPEC to be the most frequent co-infection [23].
The number of males suffering with diarrheal diseases was slightly more in comparison to females, which is similar to another study from India [30]. Our study showed that at least one infectious etiology in diarrhea is more frequently detected among children living in rural settings than urban settings. This may be due to the poor hygiene and sanitation conditions of rural population that posses a high risk of infection among children as recommended earlier.
In this study, the major etiological agent was identified to be DEC followed by Rotavirus and Shigella in the age group of 10 diarrheal episodes/day in cases with concurrent infections might be due to an aggravated effect and a number of cellular mechanisms activated simultaneously by various pathogen factors. A true understanding of pathogenesis of diarrheal disease is incomplete without the thorough understanding of biological connections of these pathogens and synergistic interaction between co-infecting pathogens. We may be able to improve our understanding of the pathogenic potential of enteric infection consistently by distinguishing between single and mixed infection.
It is also important to include healthy controls in order to compare the distribution of exposure in healthy controls compared to cases as recommended elsewhere [35]. The overall result in our study showed that 20.83% cases in non-diarrheal children also had at least one infectious agent as detected in the stool specimens. Reports originated from other studies also showed the detection of some of those agents in stool samples from human subjects with no symptoms of diarrhea [25, 26]. In the non-diarrheal group, only 3 (6.25%) subjects were found positive for multiple infections. However, it is not clear why those apparently normal individuals had the infectious agents but no symptoms of diarrhea was noticed. However, the frequency of detection of any diarrheal infectious agent or multiple agents in these apparently healthy subjects were much less, compared to those in case of the symptomatic subjects as supported on statistical analysis.
The overall finding on a broad spectrum of etiological agents of diarrhea and different combinations of concurrent infections in the pediatric patients will probably aid in planning future studies on various aspects of diarrheal diseases in this population. It is hypothesized that in cases with concurrent infections, different combinations of etiological agents might be complementing each other’s strategies of pathogenesis resulting in an increased disease severity, and other complications, which is a matter of concern.
Conclusion
This hospital-based study highlighted the overall burden of major bacterial, viral, and protozoan parasites in childhood diarrhea in the study region where multiple infections in nearly one-third of the cases pose a significant question to understand the synergistic role contributed by each associated pathogen in the overall pathogenesis of diarrheal diseases. Nevertheless, the results suggested that DEC strains such as EPEC and STEC, which are normally not screened routinely, should also be suspected in childhood diarrhea. Suspecting possible multiple infectious etiologies and diagnosis of the right causative agent(s) can help in a better pharmacological management of acute childhood diarrhea. Also it is recommended to address the issues of combined pathologies of co-existing diarrheagenic agents in experimental infection studies.
We have archived this page and will not be updating it.
You can use it for research or reference.
We have archived this page and will not be updating it.
You can use it for research or reference.
Human Campylobacter Cases
The number of Campylobacter cases reported to the NDRS declined between 2000 and 2004 (Table 15). Although Campylobacter numbers reported to the NESP were considerably lower over the fiveyear period, a similar trend was observed in NDRS. The difference between the NDRS and NESP data reflect the low frequency with which Campylobacter isolates are sent or reported from local laboratories to the provincial laboratories. NESP assesses Campylobacter isolate data under the assumption that the isolate and data flow are consistent over time in each province.
Campylobacter rates derived from the NDRS and the NESP databases are shown in Figure 24. The difference between the two databases was most apparent in British Columbia, Ontario, Québec, and Alberta. For all provinces and territories, with the exception of the Yukon and Nunavut, a decline in the rate was observed between 2000 and 2004 according to the NDRS data. The four provinces with the largest populations also had the highest rates, with British Columbia reporting a rate above 60 per 100,000 population in 2000 (NDRS).
| 2000 | 2001 | 2002 | 2003 | 2004 | ||
|---|---|---|---|---|---|---|
| NDRS | ||||||
| 2000 | 2001 | 2002 | 2003 | 2004 | |
|---|---|---|---|---|---|







