Shigella and salmonella are not found
Bacterial pathogens have evolved numerous strategies to exploit their host's cellular processes so that they can survive and persist. Often, a bacterium must adhere very tightly to the cells and mediate its effects extracellularly, or it must find a way to invade the host's cells and survive intracellularly. In either case, the pathogen hijacks the host's cytoskeleton. The cytoskeleton provides a flexible framework for the cell and is involved in mediating numerous cellular functions, from cell shape and structure to programmed cell death. Altering the host cytoskeleton is crucial for mediating pathogen adherence, invasion, and intracellular locomotion. We highlight recent advances in the pathogenesis of enteropathogenic Escherichia coli, Salmonella Typhimurium, and Shigella flexneri. Each illustrates how bacterial pathogens can exert dramatic effects on the host cytoskeleton.
Pathogenic E. coli strains remain a leading cause of severe and persistent infant diarrhea in developing countries. Although EPEC is recognized as a major diarrheal pathogen, until recently our understanding of how it causes disease lagged behind that of other pathogenic E. coli, such as enterotoxigenic E. coli or enteroinvasive E. coli.
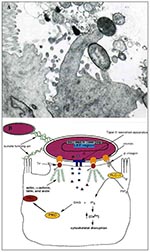
Figure 1. A. Transmission electron micrograph of an A/E lesion formed by rabbit enteropathogenic Escherichia coli (EPEC) infecting rabbit intestinal epithelial cells (micrograph provided by Dr. Ursula Heczko, Biotechnology Laboratory, University of British.
EPEC is one of a class of pathogens identified as causing attaching and effacing (A/E) lesions on intestinal cells (1). A/E pathogens typically reside on a pedestal on the surface of the host epithelial cell and ultimately cause severe disruption of the microvilli brush border (Figure 1A). Other pathogens displaying similar histopathologic features include Hafnia alvei, Citrobacter rodentium (formerly C. freundii biotype 4280), and enterohemorrhagic E. coli, the causative agent of hemolytic uremic syndrome.
The interactions between EPEC and host cells have been divided into three stages. Initial adherence to cultured epithelial cells is mediated by the formation of type IV fimbriae known as bundle forming pili (BFP) (2). While not essential for forming the characteristic A/E lesions, initial adherence helps bring the bacteria in close contact with the host cell. BFP mediate bacterial-bacterial interactions in a human intestinal organ culture model (3).
All the genes necessary for the formation of A/E lesions by EPEC are contained within a 35-kbp pathogenicity island termed the locus of enterocyte effacement (LEE) (Figure 1B) (4,5). These include the esps (E. coli-secreted protein), escs (E. coli secretion), sep (secretion of E. coli proteins), eae (E. coli attaching and effacing that encodes intimin), and tir (translocated intimin receptor) genes (6).
The second stage of EPEC pathogenesis involves the secretion of bacterial proteins, some into the host cell, including EspA, EspB, and EspD (7,8). The expression of these proteins is maximal at the host body temperature (9) and at conditions similar to those found in the gastrointestinal tract (10), which implies that they may be involved in virulence. The translocation of these proteins is essential for activating a number of signal transduction pathways (7), although their precise role in pathogenesis is not well defined. EspA makes filamentous appendages outside the bacterium and may be part of the translocation machinery involved in delivering other virulence proteins (11). EspB is translocated into the host cytosol and membrane, where it may effect changes in the host cell's signaling pathways (12). All of these effector proteins are secreted by a type-III secretion system encoded by the esc and sep genes (6). Type-III secretion systems also play an important role in other gram-negative pathogenic bacteria such as Yersinia, enabling virulence factors to be translocated directly from the bacterial cytoplasm to the host-cell membrane or cytoplasm (13).
The third stage of EPEC infection is characterized as intimate attachment with the host cell. Intimin, a 94-kDa outer membrane protein encoded by the eae gene (14), binds to a 90-kDa tyrosine phosphorylated protein in the host membrane (15). This receptor, originally thought to be a host protein, has recently been found to be of bacterial origin and has been designated as the translocated intimin receptor (Tir) (16). As the name suggests, Tir is translocated from the bacterial cell into the host membrane, where it becomes phosphorylated on one or more tyrosine residues and functions as a receptor for its binding partner, intimin. The resultant tight association is accompanied by the formation of actin pedestals up to 10 µm in length (15). Purified intimin also binds ß1 integrins, which suggests that intimin may be binding more than one receptor on the epithelial cell (17). Although integrins are not present on the apical surface of enterocytes, they are located on the apical surface of microfold cells found in Peyer's patches along the intestinal lumen (18).
The host cell undergoes a number of changes during infection by EPEC (Figure 1B). The most striking change in the cellular structure of the host cell is the formation of characteristic actin pedestals. Within 3 hours of infection by EPEC, host-cell actin, α-actinin, talin, erzin, and villin accumulate directly under the bacteria (19,20). The latter four cytoskeletal components are involved in cross-linking of actin microfilaments. Localized actin accumulation is so distinct that it forms the basis of an in vitro diagnostic test for EPEC, which uses fluorescein-tagged phalloidin to detect actin accumulation within infected cells (21). The actin pedestals are not static; instead they lengthen and shorten, resulting in apparent movement of EPEC along the host-cell surface (20). The pedestals resemble microvilli in the distribution of actin and villin (20). Microtubule and intermediate filament structures are not affected by EPEC virulence factors (19).
Intracellular calcium levels also seem to play a role in EPEC pathogenesis. EPEC-infected HEp-2 cells show significant elevation of intracellular calcium levels (22), and buffering of these levels can prevent or delay the formation of A/E lesions (23). Increases in intracellular calcium levels can result in the depolymerization of actin by villin (a calcium-dependent microvillus protein) and a breakdown of the host cytoskeleton not unlike that seen in EPEC-infected cells (24). Inositol triphosphate (IP3) is involved in the release of Ca 2+ from intracellular stores, and increased levels of IP3 (25) and inositol phosphate fluxes (26) have been observed in EPEC-infected cells. EPEC interactions with PLC-g1 HeLa epithelial cells activate a number of proteins, including phospholipase C-g1 (PLC-g1) (27). Phosphorylation of PLC-g1 leads to the IP3 and Ca 2+ fluxes mentioned above, underscoring the importance of this signaling event. Cytosolic protein kinase C also gets activated upon EPEC infection and translocates to the plasma membrane (28).
Despite the dramatic changes induced by EPEC in the cytoskeleton, there appears to be little involvement of the Rho family of small GTP-binding proteins normally involved in cytoskeletal rearrangements (29). Inhibition of Rho, Rac, and Cdc42 by compactin and Clostridium difficile ToxB, as well as dominant negative alleles, had no effect on pedestal formation by EPEC, which suggests that this pathogen uses a nontraditional mechanism to rearrange actin.
S. Typhimurium is a gram-negative bacterium that causes a variety of diseases, from gastroenteritis in humans to typhoid fever in mice. S. Typhimurium infections are contracted by oral ingestion and penetration into the intestinal epithelium before induction of systemic (invasive) disease. Invasion into the host intestinal cells results in dramatic morphologic changes to the cell that are due to exploitation of the host cytoskeleton.
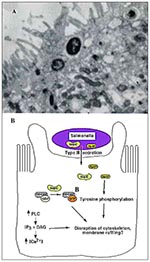
Figure 2. A. Transmission electron micrograph of Salmonella-induced membrane ruffling in polarized Caco-2 epithelial cells. B. Salmonella invasion into host epithelial cells. Salmonella secrete virulence proteins, including SopE and SptP, into host cells.
Once in close contact with the epithelium, Salmonella induces degeneration of enterocyte microvilli (30). Loss in microvillar structure is followed by profound membrane ruffling localized to the area of bacterial-host cell contact (Figure 2A) (29-31). Membrane ruffling is accompanied by profuse macropinocytosis, which leads to the internalization of bacteria into the host cells (32). The entire process occurs within minutes and when completed, Salmonella resides within membrane-bound vesicles, and the cytoskeleton returns to its normal distribution (33).
Salmonella entry into nonphagocytic epithelial cells requires several chromosomal genes (inv/spa) clustered in a pathogenicity island termed SPI1 (Salmonella pathogenicity island 1) (34). Like EPEC, SPI1 encodes a type III-secretion system and several potential virulence factors secreted by this machinery. The type III-secretion system is activated upon host-cell contact and allows export of virulence determinants directly into the host cell, where they effect bacterial uptake (35,36). Recently, SptP, a bacterial protein encoded within SPI1, has been shown to be translocated into the host epithelial cell, where it modulates the host actin cytoskeleton through its tyrosine phosphatase activity (37) (Figure 2B). Disruption of a critical Cys residue in the catalytic domain of SptP results in loss of phosphatase activity (38). It is hypothesized that SptP may function in disrupting host actin stress fibers, thereby facilitating membrane ruffling and subsequent bacterial uptake into host cells.
Other bacterial factors are not encoded next to the secretion apparatus but instead on the genome of a cryptic bacteriophage found in the Salmonella chromosome. Recently, a virulence factor encoded within this genome, SopE, has been shown to be required for efficient bacterial entry into host cells (39). SopE requires the type III-secretion system to be translocated into the host cell, where it can directly stimulate actin cytoskeletal rearrangements. It acts as a guanidine exchange factor for members of the Rho subfamily of small GTPases. sopE mutants exhibit less extensive actin cytoskeletal rearrangements upon entry into epithelial cells than do wild-type Salmonella (40). This discovery clearly illustrates how pathogens (which contain no primary sequence homology with host proteins) can craftily subvert the host's own signaling machinery within the cell by mimicking host proteins.
The massive restructuring of the host cytoskeletal components during Salmonella entry requires many host factors. A Rho subfamily member, Cdc42, is needed for mediating bacterial uptake through membrane ruffling (41). It is believed that the guanidine exchange activity of SopE is responsible for the stimulation of Cdc42 in the host. The pathogen also activates host PLC upon bacterial contact, leading to the production of two second messengers, which further initiate signaling events (42). As a consequence, the host cell's Ca 2+ levels are altered to trigger cytoskeletal rearrangements resulting in Salmonella invasion. Although EPEC and Salmonella use some of the same signaling components (PLC, Ca 2+ fluxes), the cytoskeletal changes induced in the host cell by each pathogen are quite different. This could be the result of different upstream or downstream effectors in the signaling pathway. Several cytoskeletal components involved in invasion have been identified. These include α-actinin, tropomyosin, ezrin, and talin (19). The specific roles of these proteins in Salmonella invasion are not defined.
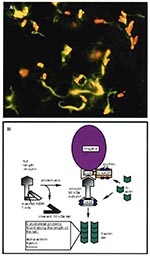
Figure 3. A. Immunofluorescence micrograph showing Shigella (red) propelling itself through the cytoplasm by polymerizing actin (green) (Philippe Sansonetti, Institut Pasteur, reprinted with permission from Trends in Microbiology, 1996). B. Shigella-mediated cytoskeletal rearrangements.
S. flexneri, a gram-negative bacillus that causes bacillary dysentery in humans, directs its own uptake into the colonic mucosa through membrane ruffling and macropinocytosis in a manner similar to Salmonella uptake (43,44). After engulfment, the pathogen is surrounded by a membrane-bound vacuole within the host. Unlike Salmonella, however, Shigella rapidly lyses the surrounding vacuole and is released into the cytosol, where it grows and divides (45). Once the microbe has escaped from the vacuole, it quickly becomes coated with filamentous actin and ultimately forms an actin tail at one pole of the bacterium (Figure 3A) (46,47). This actin polymerization propels the bacterium through the cytoplasm at speeds reaching 0.4 µm/sec (48). When the pathogen reaches the plasma membrane of the cell, it forms a long protrusion into the neighboring cell, which subsequently internalizes the microbe (49). The bacterium again breaks out of the vacuole, thereby starting a new cycle of infection in a new host cell (50). This process allows Shigella to move from cell to cell without ever contacting the extracellular milieu.
Analysis of mutants deficient in intracellular motility and cell-to-cell spread has identified a bacterial gene, icsA, necessary for Shigella locomotion (46,51,52). IcsA (also called VirG) is a 120-kDa outer membrane protein that hydrolyzes ATP and is localized to one pole of the bacterium, at the junction between the microbe and the actin tail (Figure 3B) (53). IcsA expression on the surface of Shigella is sufficient to direct actin-based motility (54,55). In fact, E. coli expressing IcsA can synthesize actin tails in cytoplasmic extracts (54,55).
During infection, IcsA is also detected as a 95-kDa amino-terminal fragment of the 120-kDa full-length protein (53). This proteolytic cleavage of IcsA is due to a bacterial protease, SopA (IcsP) (56,57). Cleavage is required for polarized distribution of IcsA on the bacterial surface and for proper actin-based motility of Shigella in infected cells (56-58).
IcsA expression on the Shigella surface promotes rapid accumulation of actin around the bacterium. Following bacterial division and IcsA polarization, actin tails begin to form on one end of the bacterium. Several host cytoskeletal proteins are involved in tail formation, including α-actinin (48), filamin (59), fimbrin (59), vasodilator-stimulated phosphoprotein (VASP) (60), vinculin (49,61), and neural-Wiskott-Aldrich syndrome protein (N-WASP) (63). Of these proteins, only vinculin and N-WASP are able to directly bind IcsA (61,62).
Shigella infection results in the cleavage of intact vinculin (120 kDa) to produce a 90-kDa fragment (63). This proteolysis unmasks an actin-based motility 1 site on vinculin, which contains a polyproline region capable of binding VASP. VASP recruitment to the bacterial surface in turn allows the recruitment of other cytoskeletal proteins, such as actin and profilin, and forms the basis of an actin-based motor for Shigella movement.
Recently, N-WASP was shown to be required for Shigella motility (62); like vinculin, it can bind IcsA directly. It is possible that N-WASP, in addition to VASP, can recruit profilin and actin to the surface of Shigella, thereby mediating actin polymerization. Furthermore, N-WASP contains an actin depolymerization factor/cofilin homologous region, which could be used for severing actin filaments at the pointed ends and increasing the monomeric actin concentration. The precise mechanisms involved in Shigella-driven actin polymerization, however, are unclear.
Bacterial pathogens have evolved several mechanisms to hijack host-cell signaling machinery and disrupt the cytoskeleton. EPEC mediates its effects on the host cell from the cellular surface. It secretes its own receptor, Tir, into the host and then binds intimately to it by its outer membrane protein, intimin. Tir-intimin binding results in a dramatic reorganization of the cytoskeleton to form the pedestal upon which EPEC resides. Salmonella, on the other hand, actively invades intestinal epithelial cells by inducing membrane ruffling and macropinocytosis. Invasion is dependent on the secretion of virulence proteins, including SptP and SopE, into the host cell, and mediates its effects on the host from within a membrane-bound vesicle. Shigella is also an invasive pathogen but lyses the phagocytic vacuole and initiates intracellular actin-based locomotion to spread from cell to cell in the cytoplasm. This motility is dependent on the bacterial outer membrane protein IcsA, which recruits several actin-associated proteins to the bacterial surface. Despite the outward differences between each mode of pathogenesis, EPEC, Salmonella, and Shigella have effectively managed to subvert the host cytoskeleton for their own purposes and cause substantial diarrheal disease.
While the food we eat in Canada is generally very safe, sometimes it may carry bacteria that can make us sick, like Shigella.
What is Shigella?
Shigella bacteria are found naturally only in the intestines of humans and high primates, mostly in capture (zoos). They are usually transferred to other people, food or water when people do not wash their hands after using the toilet and then touch something or someone else.
People who are infected with Shigella can become ill with shigellosis, an acute intestinal illness. Like other foodborne illnesses, the symptoms of shigellosis can feel like stomach flu, but they can also develop into serious illness with long-lasting effects.

How do people get sick?
Shigellosis, the infection caused by Shigella, can be transmitted through person-to-person contact, poor-quality drinking water, contaminated surfaces or contaminated food. Shigella can also be transferred by flies, which breed in contaminated feces (stool), then contaminate food and surfaces. People can be carriers of Shigella bacteria without knowing it, then spread the bacteria to food, surfaces or other people.
Intestinal illness can be caused by viruses, bacteria or parasites, and usually involves vomiting and diarrhea. People often call it the flu, though it is in no way related to the influenza virus, which causes respiratory illness.
Outbreaks of shigellosis are more common in places where hygiene practices are poor, and under conditions of crowding, like jails, daycare centres, and refugee camps.
Shigella is not naturally present on foods. Food is most often contaminated with Shigella from water polluted by human sewage. Food can also become contaminated if it is handled by a person infected with Shigella or by cross-contamination because of unsanitary food handling practices.
Foods that can become contaminated with Shigella bacteria because of unsafe handling can include:
- raw oysters and shellfish harvested from contaminated waters
- vegetables harvested from fields contaminated with sewage
- salads (pasta, potato, shrimp, tuna, chicken, turkey, macaroni, fruit, lettuce, vegetable)
- water contaminated with sewage
- chopped turkey
- rice balls
- beans
- pudding
- deli meats
- unpasteurized milk
You can also be exposed to Shigella bacteria (or spread it) by:
- not washing your hands with soap after using the bathroom
- not washing your hands with soap before handling food
- direct person-to-person contact, including contact with hands that were not washed properly after using the bathroom
- bathing in contaminated waters
What are the symptoms and treatment?
Shigellosis is most often spread from person-to-person. About 20 per cent of shigellosis infections come directly from contaminated food and water.
People with shigellosis can experience a wide range of symptoms. Some do not get sick at all, though they can still spread the infection to others. Others feel as though they have a bad case of stomach flu. A rare few become seriously ill and must be hospitalized.
Most people with shigellosis develop the following symptoms one to three days after being infected with Shigella bacteria (though symptoms can appear as late as seven days after infection):
- diarrhea (watery and often bloody)
- fever
- nausea
- vomiting
- abdominal pains
- stomach cramps
The illness usually lasts between five and seven days, and most people recover fully, though it may take several weeks to months before bowel habits return to normal. As with any disease causing diarrhea or vomiting, people infected should drink plenty of liquids to replace lost body fluids and prevent dehydration. Although anyone can get shigellosis, pregnant women, people with weakened immune systems, young children and older adults are most at risk for developing serious complications like septicemia (an infection of the bloodstream).
While long-term consequences are rare, a small number of people infected with Shigella flexneri may develop Reiter's syndrome, a condition that develops in response to an infection in another part of the body and can lead to chronic arthritis.
How do I avoid getting sick?
Foods contaminated with Shigella look, smell and taste normal. The good news is, Shigella and many other harmful bacteria can be killed by cooking and preparing (ready-to-eat) food properly.
These tips will help protect you and your family from Shigella:
- Always wash your hands for 20 seconds with soap after using the bathroom.
- Wash your hands well with soap before handling any food. Be sure to wash your hands, cutting boards, counters, knives and other utensils after preparing raw foods. This will avoid cross contamination.
- Cook food to a safe internal temperature using a digital thermometer.
- Buy shellfish from reputable suppliers.
- Cook shellfish thoroughly before eating, especially oysters.
- Drink water from a safe (treated or boiled) water supply.
- Eat and drink only pasteurized juice, cider, milk and milk products. Mother's milk is the safest food for infants.
- When travelling, in particular in developing countries, drink water from a safe (treated or boiled) source. Eat only cooked hot food. Eat only fruit that can be peeled.
- Wash raw fruits and vegetables thoroughly with clean, safe running water before you prepare and eat them. Use a brush to scrub produce with firm or rough surfaces, like oranges, cantaloupes, potatoes and carrots.
- If you have been diagnosed with shigellosis or any other gastrointestinal illness, do not prepare food or pour water for other people.
Also, these safe food practices will reduce your risk of contracting shigellosis and other foodborne illnesses.
What does the Government do to protect me?
In Canada, several government organizations work together every day to keep your food safe:
- Health Canada makes food safety standards and policies to help minimize the risk of foodborne illnesses.
- The Canadian Food Inspection Agency (CFIA) enforces these policies and standards and carries out inspections to make sure the food industry meets its food safety responsibilities. The CFIA works with Health Canada to make sure that foodborne illness is detected early and warnings go out to the public quickly.
- The Public Health Agency of Canada studies the incidence and causes of diseases in Canada, conducts outbreak surveillance, and coordinates outbreak response.
The Government of Canada works very hard to protect your health and safety:
- We are carrying out a five-year Food and Consumer Safety Action Plan, to strengthen and modernize Canada's safety system and make sure you can have confidence in the quality and safety of the food, health and consumer products you buy.
- We are investing $75 million more in Canada's food safety system (on top of the $113 million committed in 2008) to hire more inspectors, update lab technology, and improve communication with Canadians.
- We support and participate in public awareness campaigns about safe food practices, like the Canadian Partnership for Consumer Food Safety Education'sBe Food Safe program, which encourages Canadian consumers to think of food safety at every step of the food handling process, from shopping for groceries to re-heating leftovers.
You may feel a sharp cramp in your stomach and lower abdomen. Then, you may have the urge to use the bathroom -- as many as 10 to 30 times a day when you have shigellosis, a type of food poisoning.
Caused by a group of bacteria called shigella, this infection can cause belly pain, fever, and watery or bloody diarrhea.
The illness is common among young children, who usually get infected at day care or school. You might also get shigellosis while you’re visiting developing countries where poor hygiene could cause traveler’s diarrhea.
The disease usually goes away in 5 to 7 days with rest and fluids. But in severe cases, you may need to go to the hospital.
Shigellosis is common in the United States with about a half-million cases every year. It’s far more deadly in poorer countries (about 165 million cases and about 1 million deaths worldwide every year).
How Do You Get Shigellosis?
The Shigella bacteria pass through your stomach and then multiply in your small intestines. They then spread into your large intestines (also known as colon), causing cramping in that part of your body, along with diarrhea.
Shigella leaves the body through human feces. The disease spreads when bacteria from the stool of the sick person go to the mouth of another person.
You may be wondering: How on earth does that happen? Shigella spreads more easily than you might think. Here are some ways:
Touching objects. For example, you may change the diaper of a child who has shigellosis. If you don’t wash your hands thoroughly, you could leave the bacteria behind on objects you touch next, such as changing tables, toys, and doorknobs.
The people who touch those infected surfaces can get infected -- especially if they touch their mouths or swallow something using their contaminated hands.
Eating. People handling or preparing your food may have shigellosis. If their hands aren’t clean, your food may be tainted. Or your fruits and vegetables may have been growing on a field that has been contaminated with human feces.
Swallowing water. You could go swimming in pool or pond and get water in your mouth that’s been contaminated by feces.
Sexual contact. You could get exposed during sexual activity when it involves oral-anal contact.
What Are the Symptoms?
The main symptom is diarrhea. The stools may be bloody or contain mucus. Other symptoms you or your child may get include:
- Nausea
- Vomiting
- Fever
- Cramping in your stomach and abdominal area
- Tenesmus (the feeling that you need to go to the bathroom even when there is nothing left in your intestines)
For people with mild cases, you can expect your symptoms to clear up without drugs in a week.
But shigellosis can be worse on seniors, infants or people who have chronic illnesses that have weakened their immune systems (HIV, for example).
You should call your doctor if:
- Your diarrhea is severe, especially if you spot blood or mucus
- You have a fever
- You have signs of dehydration such as dry mouth, lips or lightheadedness
Not everyone with shigellosis get symptoms. Although you may not have symptoms, you are still infectious and could spread the disease to other people.
Does It Cause Other Problems?
You could have lingering effects after a shigella infection, though such cases are rare. Problems may include:
Dehydration. This is when you don’t have enough fluid in your system. You could be lightheaded, dizzy, lack tears, and sunken eyes. Watch for dry diapers in children.
Post-infectious arthritis. This is joint pain (in ankles, knees, feet, hips). You could also get eye irritations and painful urination. This occurs to about 2% of people who get infected with shigella flexneri, a type of shigella bacteria.
Bloodstream infection. When the lining of the intestines gets damaged during the sickness, shigella or other germs in your gut could infect your bloodstream. These infections are more common among people with other illnesses, such as HIV, cancer, or malnutrition.
Hemolytic-uremic syndrome (HUS): This infection produces a toxin that destroys red blood cells, which are cells in your blood that carry oxygen.
Seizures: This is more commonly seen in young children. Call 911 at once if your child has a seizure.
How Is it Diagnosed?
Since there are many causes of diarrhea, a lab test may be needed to figure out whether you have shigellosis. Your doctor may ask you to give a stool sample to see whether you have shigella bacteria.
The lab can run more tests to find out which antibiotic would be the most effective.
What's the Treatment?
In most cases, you can recover from shigellosis by resting and drinking fluids to replace what you’ve lost from diarrhea.
Avoid drugs that stop diarrhea or slow down the gut. Drugs such as diphenoxylate with atropine (Lomotil) or loperamide (Imodium) can make shigellosis worse.
In severe cases, your doctor may prescribe antibiotics to shorten the illness. This may be for seniors, infants, or people who have other diseases. Some shigella bacteria are resistant to antibiotics, so the treatment may not work.
Tell your doctor if prescription antibiotics don’t make you feel better after you’ve taken them for several days.
Can I Prevent Shigellosis?
There is no vaccine or cure, so the key is good hygiene.
Wash your hands well with warm water and soap, especially after using the bathroom or changing diapers, and before preparing food or eating. Also make sure young children wash their hands after using bathroom.
Some other tips:
- Keep children with diarrhea out of day care or school.
- Don’t drink water from a pool, lake, or pond.
- Eat only boiled, cooked, or peeled food while traveling abroad.
- Wash your hands even more when traveling abroad.
- Wrap up soiled diapers properly and put them in a trash can.
- Avoid having sex with someone who had diarrhea recently.
Harvard Health Publications: “Shigellosis.”
Mayo Clinic: “Shigella Infection.”
Centers for Disease Control and Prevention: “Traveler’s Diarrhea.”
National Institute of Allergy and Infectious Diseases: “Shigellosis.”
California Department of Public Health: “Shigellosis.”
UpToDate: “Shigella infection: Treatment and prevention in adults,” “Shigella infection: Clinical manifestations and diagnosis.”
Читайте также:


