Created to you вирус
The feat has triggered new warnings about controversial research
- By Helen Branswell, STAT on July 10, 2017

"data-newsletterpromo-image="https://static.scientificamerican.com/sciam/cache/file/CF54EB21-65FD-4978-9EEF80245C772996_source.jpg"data-newsletterpromo-button-text="Sign Up"data-newsletterpromo-button-link="https://www.scientificamerican.com/page/newsletter-sign-up/?origincode=2018_sciam_ArticlePromo_NewsletterSignUp"name="articleBody" itemprop="articleBody">
For years there have been warnings that advances in science could make it possible to cook up killer diseases in laboratories and unleash them on the world.
This week came news that scientists at the University of Alberta have put together from scratch a relative of the smallpox virus — and a reminder that the threat of deadly viruses created by humans is more than theoretical.
The smallpox virus, which triggered brutal disease for centuries, was declared eradicated in 1980 after a successful global effort to end its reign of terror. But some scientists fear that it could be revived through what’s known as synthetic biology — the ability to make a virus by putting together by the recipe outlined in its genetic code.
The horsepox virus the Canadian team created is not a threat to human health — or even the health of horses — should it ever escape from a lab. And it’s not the first virus created by putting pieces of DNA together in the right sequence.
Still, the news that a team headed by David Evans, a professor of medical microbiology and immunology, had accomplished this feat — at a relatively low cost of about $100,000 plus labor — was a bit of a wakeup call. The news was first reported Thursday in Science Magazine.
“This is an example of what modern technologies can do,” noted Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases.
The warnings about the implications of synthetic biology have echoed since Eckard Wimmer of the State University of New York at Stony Brook — now Stony Brook University — reported in 2002 that he and his team had made a poliovirus from scratch.
Polioviruses are small in comparison with poxviruses, and a far less complex task. But scientists watching this field feared it was only a matter of time before the obstacles to creating other viruses were surmounted.
Evans, who is a member of a World Health Organization advisory committee that reviews applications to do smallpox-related research, said he had warned the committee the science was there.
“Really, nobody in the field is surprised about that and we have been telling WHO that for years now,” he told STAT. “This technology is quite possible. And my sense is that we need to just accept that and move on.”
Evans wanted to prove the point. But he also had a bigger goal in mind. He is trying to develop cancer vaccines and wants to use a vaccinia virus — another relative of smallpox, often used in research — as a way to introduce cancer fighters to the immune system. Learning how to make vaccinia viruses synthetically would speed this work.
Meanwhile, a pharmaceutical company named Tonix was interested in making a newer, better smallpox vaccine, using the horsepox virus as its delivery mechanism. Evans and his team agreed to make horsepox — which seems to have disappeared in nature — for the company. Tonix funded the research.
Evans, who is vice dean of research at the University of Alberta, sought and received approval from the university to do the work.
Had he been in the United States, though, it is not clear if he could have performed this work. The proposal would have certainly gone through more approval processes, Fauci said, adding he did not know if the work would have received a green light.
Those levels of approvals are the result of a protracted and at times heated debate in the U.S. about research that can be considered to pose security threats. Generally referred to as DURC — dual use research of concern — this is otherwise valid research that could be used by rogue agents to weaponize pathogens.
The debate was triggered when, in 2011, a Dutch scientist using U.S. government funding manipulated H7N9 bird flu viruses until they developed the ability to spread readily among ferrets. Ferrets are a stand-in for humans in flu research because viruses that can infect them can infect people.
Marc Lipsitch, a professor of epidemiology at Harvard University, was a vocal critic of the flu research and has been active in the debate since over restricting work that could be used for nefarious purposes.
Asked about Evans’s work, he expressed concern not so much about the work itself but about the fact the world now knows about it.
“Demonstrating this can be done — and then writing newspaper articles about it and Science magazine articles — will get the attention of people who might want to use it for the wrong reasons and they might have never known about that,” Lipsitch said.
The horsepox work hasn’t yet been published. In fact, it has been turned down by two journals so far. And Evans admits he’s struggling with how much detail to include in the manuscript when he next submits it.
“I don’t want to put in detailed instructions on how to do this. If you’re knowledgeable in the field, you’ll know enough to replicate it. And if you’re not knowledgeable in the field, you probably shouldn’t be doing it anyway, right?” he noted.
Lipsitch found Evans’s attitude reassuring, but was perhaps less sanguine about the fact that this research shows that as complicated science becomes easier to do, government control over it may lessen.
The rules the National Institutes of Health have put in place relate to work that is done with its funding. But Evans did the work outside the U.S. and with private money.
“As potentially dangerous work gets cheaper and easier, it becomes harder to control for all sorts of reasons — including the fact that the legal reach of government is greater for things that they finance than for things they don’t finance,” Lipsitch said.
Republished with permission from STAT. This article originally appeared on July 7, 2017

Helen Branswell is STAT's infectious diseases and public health reporter. She comes from the Canadian Press, where she was the medical reporter for the past 15 years. Helen cut her infectious diseases teeth during Toronto's SARS outbreak in 2003 and spent the summer of 2004 embedded at the US Centers for Disease Control and Prevention. In 2010-11 she was a Nieman Global Health Fellow at Harvard, where she focused on polio eradication. Warning: Helen asks lots of questions.
Credit: Nick Higgins
STAT delivers fast, deep, and tough-minded journalism. We take you inside science labs and hospitals, biotech boardrooms, and political backrooms. We dissect crucial discoveries. We examine controversies and puncture hype. We hold individuals and institutions accountable. We introduce you to the power brokers and personalities who are driving a revolution in human health. These are the stories that matter to us all.
The persistent myth can be put to bed.
Editor's note: On April 16, news came out that the U.S. government said it was investigating the possibility that the novel coronavirus may have somehow escaped from a lab, though experts still think the possibility that it was engineered is unlikely. This Live Science report explores the origin of SARS-CoV-2.
As the novel coronavirus causing COVID-19 spreads across the globe, with cases surpassing 284,000 worldwide today (March 20), misinformation is spreading almost as fast.
One persistent myth is that this virus, called SARS-CoV-2, was made by scientists and escaped from a lab in Wuhan, China, where the outbreak began.
A new analysis of SARS-CoV-2 may finally put that latter idea to bed. A group of researchers compared the genome of this novel coronavirus with the seven other coronaviruses known to infect humans: SARS, MERS and SARS-CoV-2, which can cause severe disease; along with HKU1, NL63, OC43 and 229E, which typically cause just mild symptoms, the researchers wrote March 17 in the journal Nature Medicine.
"Our analyses clearly show that SARS-CoV-2 is not a laboratory construct or a purposefully manipulated virus," they write in the journal article.
Kristian Andersen, an associate professor of immunology and microbiology at Scripps Research, and his colleagues looked at the genetic template for the spike proteins that protrude from the surface of the virus. The coronavirus uses these spikes to grab the outer walls of its host's cells and then enter those cells. They specifically looked at the gene sequences responsible for two key features of these spike proteins: the grabber, called the receptor-binding domain, that hooks onto host cells; and the so-called cleavage site that allows the virus to open and enter those cells.
That analysis showed that the "hook" part of the spike had evolved to target a receptor on the outside of human cells called ACE2, which is involved in blood pressure regulation. It is so effective at attaching to human cells that the researchers said the spike proteins were the result of natural selection and not genetic engineering.
Here's why: SARS-CoV-2 is very closely related to the virus that causes severe acute respiratory syndrome (SARS), which fanned across the globe nearly 20 years ago. Scientists have studied how SARS-CoV differs from SARS-CoV-2 — with several key letter changes in the genetic code. Yet in computer simulations, the mutations in SARS-CoV-2 don't seem to work very well at helping the virus bind to human cells. If scientists had deliberately engineered this virus, they wouldn't have chosen mutations that computer models suggest won't work. But it turns out, nature is smarter than scientists, and the novel coronavirus found a way to mutate that was better — and completely different— from anything scientists could have created, the study found.
Another nail in the "escaped from evil lab" theory? The overall molecular structure of this virus is distinct from the known coronaviruses and instead most closely resembles viruses found in bats and pangolins that had been little studied and never known to cause humans any harm.
"If someone were seeking to engineer a new coronavirus as a pathogen, they would have constructed it from the backbone of a virus known to cause illness," according to a statement from Scripps.
Where did the virus come from? The research group came up with two possible scenarios for the origin of SARS-CoV-2 in humans. One scenario follows the origin stories for a few other recent coronaviruses that have wreaked havoc in human populations. In that scenario, we contracted the virus directly from an animal — civets in the case of SARS and camels in the case of Middle East respiratory syndrome (MERS). In the case of SARS-CoV-2, the researchers suggest that animal was a bat, which transmitted the virus to another intermediate animal (possibly a pangolin, some scientists have said) that brought the virus to humans.
In that possible scenario, the genetic features that make the new coronavirus so effective at infecting human cells (its pathogenic powers) would have been in place before hopping to humans.
In the other scenario, those pathogenic features would have evolved only after the virus jumped from its animal host to humans. Some coronaviruses that originated in pangolins have a "hook structure" (that receptor binding domain) similar to that of SARS-CoV-2. In that way, a pangolin either directly or indirectly passed its virus onto a human host. Then, once inside a human host, the virus could have evolved to have its other stealth feature — the cleavage site that lets it easily break into human cells. Once it developed that capacity, the researchers said, the coronavirus would be even more capable of spreading between people.
All of this technical detail could help scientists forecast the future of this pandemic. If the virus did enter human cells in a pathogenic form, that raises the probability of future outbreaks. The virus could still be circulating in the animal population and might again jump to humans, ready to cause an outbreak. But the chances of such future outbreaks are lower if the virus must first enter the human population and then evolve the pathogenic properties, the researchers said.
Coronavirus science and news
The one-month trial gives you access to all of the educational site's 9,000 activities in reading, science, math and art. Keep your child busy and learning while we are all stuck indoors.
View Deal
Scientists took conspiracy theories about SARS-CoV-2’s origins seriously, and debunked them
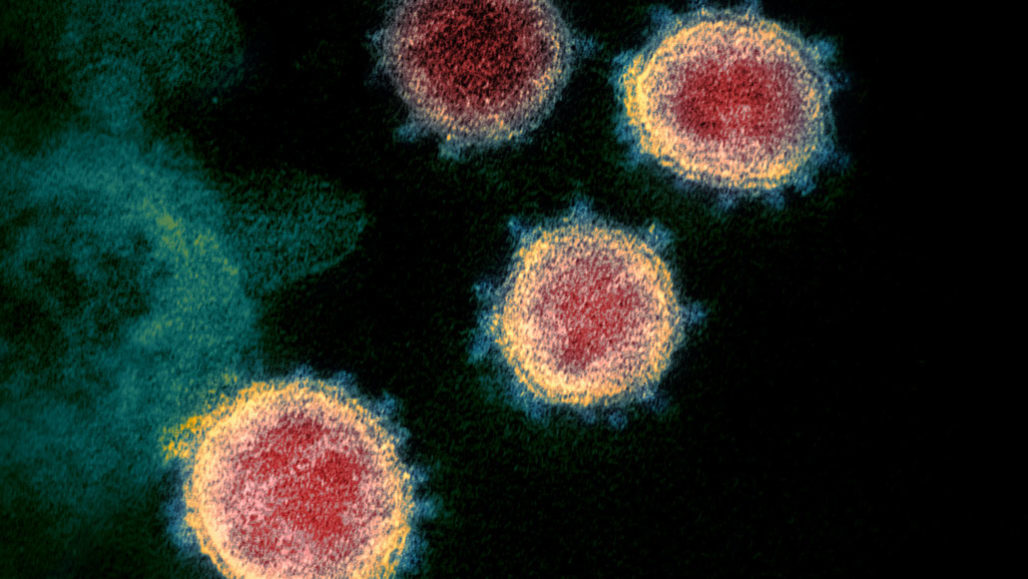
The SARS-CoV-2 virus (seen in this transmission electron microscope image of virus isolated from a U.S. patient), which causes COVID-19, was rumored to be human-made, but scientists have now debunked that theory.
March 26, 2020 at 6:00 am
The coronavirus pandemic circling the globe is caused by a natural virus, not one made in a lab, a new study says.
The virus’s genetic makeup reveals that SARS-CoV-2 isn’t a mishmash of known viruses, as might be expected if it were human-made. And it has unusual features that have only recently been identified in scaly anteaters called pangolins, evidence that the virus came from nature, Kristian Andersen and his colleagues report March 17 in Nature Medicine.
When Andersen, an infectious disease researcher at the Scripps Research Institute in La Jolla, Calif., first heard about the coronavirus causing an outbreak in China, he wondered where the virus came from. Initially, researchers thought the virus was being spread by repeated infections jumping from animals in a seafood market in Wuhan, China, into humans and then being passed person to person. Analysis from other researchers has since suggested that the virus probably jumped only once from an animal into a person and has been spread human to human since about mid-November (SN: 3/4/20).
But shortly after the virus’s genetic makeup was revealed in early January, rumors began bubbling up that maybe the virus was engineered in a lab and either intentionally or accidentally released.

Headlines and summaries of the latest Science News articles, delivered to your inbox
An unfortunate coincidence fueled conspiracy theorists, says Robert Garry, a virologist at Tulane University in New Orleans. The Wuhan Institute of Virology is “in very close proximity to” the seafood market, and has conducted research on viruses, including coronaviruses, found in bats that have potential to cause disease in people. “That led people to think that, oh, it escaped and went down the sewers, or somebody walked out of their lab and went over to the market or something,” Garry says.
Accidental releases of viruses, including SARS, have happened from other labs in the past. So “this is not something you can just dismiss out of hand,” Andersen says. “That would be foolish.”
Andersen assembled a team of evolutionary biologists and virologists, including Garry, from several countries to analyze the virus for clues that it could have been human-made, or grown in and accidentally released from a lab.
“We said, ‘Let’s take this theory — of which there are multiple different versions — that the virus has a non-natural origin … as a serious potential hypothesis,’ ” Andersen says.
Meeting via Slack and other virtual portals, the researchers analyzed the virus’s genetic makeup, or RNA sequence, for clues about its origin.
It was clear “almost overnight” that the virus wasn’t human-made, Andersen says. Anyone hoping to create a virus would need to work with already known viruses and engineer them to have desired properties.
But the SARS-CoV-2 virus has components that differ from those of previously known viruses, so they had to come from an unknown virus or viruses in nature. “Genetic data irrefutably show that SARS-CoV-2 is not derived from any previously used virus backbone,” Andersen and colleagues write in the study.
“This is not a virus somebody would have conceived of and cobbled together. It has too many distinct features, some of which are counterintuitive,” Garry says. “You wouldn’t do this if you were trying to make a more deadly virus.”
Other scientists agree. “We see absolutely no evidence that the virus has been engineered or purposely released,” says Emma Hodcroft, a molecular epidemiologist at the University of Basel in Switzerland. She was not part of Andersen’s group, but is a member of a team of scientists with Nextstrain.org that is tracking small genetic changes in the coronavirus to learn more about how it is spreading around the world.
That finding debunks a widely disputed analysis, posted at bioRxiv.org before peer review, that claimed to find bits of HIV in the coronavirus, Hodcroft says. Other scientists quickly pointed out flaws in the study and the authors retracted the report, but not before it fueled the notion that the virus was engineered.
Some stretches of the virus’s genetic material are similar to HIV, but that’s something that stems from those viruses sharing a common ancestor during evolution, Hodcroft says. “Essentially their claim was the same as me taking a copy of the Odyssey and saying, ‘Oh, this has the word the in it,’ and then opening another book, seeing the word the in it and saying, ‘Oh my gosh, it’s the same word, there must be parts of the Odyssey in this other book,” she says. “It was a really misleading claim and really bad science.”
Andersen’s group next set out to determine whether the virus could have been accidentally released from a lab. That’s a real possibility because researchers in many places are working with coronaviruses that have potential to infect humans, he says. “Stuff comes out of the lab sometimes, almost always accidentally,” he says.
A couple of unexpected features of the virus caught the researchers’ eyes, Andersen says. In particular, the gene encoding the coronavirus’s spike protein has 12 extra RNA building blocks, or nucleotides, stuck in it.
This spike protein protrudes from the virus’ surface and allows the virus to latch onto and enter human cells. That insertion of RNA building blocks adds four amino acids to the spike protein, and creates a site in the protein for an enzyme called furin to cut. Furin is made in human cells, and cleaves proteins only at spots where a particular combination of amino acids is found, like the one created by the insertion. SARS and other SARS-like viruses don’t have those cutting sites.

Scientists and journalists share a core belief in questioning, observing and verifying to reach the truth. Science News reports on crucial research and discovery across science disciplines. We need your financial support to make it happen – every contribution makes a difference.
Finding the furin cutting site was a surprise: “That was an aha moment and an uh-oh moment,” Garry says. When bird influenza viruses acquire the ability to be cut by furin, the viruses often become more easily transmissible. The insertion also created places where sugar molecules could be fastened to the spike protein, creating a shield to protect the virus from the immune system.
The COVID-19 virus’ spike protein also binds more tightly to a protein on human cells called ACE2 than SARS does (SN: 3/10/20). Tighter binding may allow SARS-CoV-2 to more easily infect cells. Together, those features may account for why COVID-19 is so contagious (SN: 3/13/20).
“It’s very peculiar, these two features,” Andersen says. “How do we explain how this came about? I’ve got to be honest. I was skeptical [that it was natural]. This could have happened in tissue culture” in a lab, where viruses may acquire mutations as they replicate many times in lab dishes. In nature, viruses carrying some of those mutations might be weeded out by natural selection but might persist in lab dishes where even feeble viruses don’t have to fight hard for survival.
But then the researchers compared SARS-CoV-2 with other coronaviruses recently found in nature, including in bats and pangolins. “It looks like SARS-CoV-2 could be a mix of bat and pangolin viruses,” Garry says.
Viruses, especially RNA viruses such as coronaviruses, often swap genes in nature. Finding genes related to the pangolin viruses was especially reassuring because those viruses’ genetic makeup wasn’t known until after SARS-CoV-2’s discovery, making it unlikely anyone was working with them in a lab, he says.

Coronaviruses that infect pangolins gave researchers important clues that the SARS-CoV-2 virus is natural. 2630ben/iStock/Getty Images Plus
In particular, pangolins also have the amino acids that cause the tight binding of the spike protein to ACE2, the team found. “So clearly, this is something that can happen in nature,” Andersen says. “I thought that was very important little clue. It shows there’s no mystery about its tighter binding to the human [protein] because pangolins do it, too.”
The sugar-attachment sites were another clue that the virus is natural, Andersen says. The sugars create a “mucin shield” that protects the virus from an immune system attack. But lab tissue culture dishes don’t have immune systems, making it unlikely that such an adaptation would arise from growing the virus in a lab. “That sort of explained away the tissue-culture hypothesis,” he says.
Similarity of SARS-CoV-2 to bat and pangolin viruses is some of the best evidence that the virus is natural, Hodcroft says. “This was just another animal spillover into humans,” she says. “It’s really the most simple explanation for what we see.” Researchers still aren’t sure exactly which animal was the source.
Andersen says the analysis probably won’t lay conspiracy theories to rest. Still, he thinks the analysis was worth doing. “I was myself skeptical at the beginning and I kept flipping back and forth,” Andersen says, but he’s now convinced. “All the data show it’s natural.”
Questions or comments on this article? E-mail us at feedback@sciencenews.org

Tina Hesman Saey is the senior staff writer and reports on molecular biology. She has a Ph.D. in molecular genetics from Washington University in St. Louis and a master’s degree in science journalism from Boston University.








Science News
Science News was founded in 1921 as an independent, nonprofit source of accurate information on the latest news of science, medicine and technology. Today, our mission remains the same: to empower people to evaluate the news and the world around them. It is published by Society for Science & the Public, a nonprofit 501(c)(3) membership organization dedicated to public engagement in scientific research and education.
Log in
Subscribers, enter your e-mail address to access the Science News archives.
Share this with
These are external links and will open in a new window
These are external links and will open in a new window
Close share panel
Coronavirus is spreading around the world, but there are still no vaccines to protect the body against the disease it causes, Covid-19.
Medical researchers are working hard to change that.
Why is a coronavirus vaccine important?
The virus spreads easily and the majority of the world's population is still vulnerable to it. A vaccine would provide some protection by training people's immune systems to fight the virus so they should not become sick.
This would allow lockdowns to be lifted more safely, and social distancing to be relaxed.
What sort of progress is being made?
Research is happening at breakneck speed. About 80 groups around the world are researching vaccines and some are now entering clinical trials.
- The first human trial for a vaccine was announced last month by scientists in Seattle. Unusually, they are skipping any animal research to test its safety or effectiveness
- In Oxford, the first human trial in Europe has started with more than 800 recruits - half will receive the Covid-19 vaccine and the rest a control vaccine which protects against meningitis but not coronavirus
- Pharmaceutical giants Sanofi and GSK have teamed up to develop a vaccine
- Australian scientists have begun injecting ferrets with two potential vaccines. It is the first comprehensive pre-clinical trial involving animals, and the researchers hope to test humans by the end of April
However, no-one know how effective any of these vaccines will be.
When will we have a coronavirus vaccine?
A vaccine would normally take years, if not decades, to develop. Researchers hope to achieve the same amount of work in only a few months.
Most experts think a vaccine is likely to become available by mid-2021, about 12-18 months after the new virus, known officially as Sars-CoV-2, first emerged.
That would be a huge scientific feat and there are no guarantees it will work.
Four coronaviruses already circulate in human beings. They cause common cold symptoms and we don't have vaccines for any of them.
What do I need to know about the coronavirus?
- A SIMPLE GUIDE: How do I protect myself?
- AVOIDING CONTACT: The rules on self-isolation and exercise
- HOPE AND LOSS: Your coronavirus stories
- LOOK-UP TOOL: Check cases in your area
- VIDEO: The 20-second hand wash
What still needs to be done?
Multiple research groups have designed potential vaccines, however, there is much more work to do.
- Trials need to show the vaccine is safe. It would not be useful if it caused more problems than the disease
- Clinical trials will also need to show the vaccine provokes an immune response which would protect people from getting sick
- A way of producing the vaccine on a huge scale must be developed for the billions of potential doses
- Medicines regulators must approve it before it can be given
- Finally there will be the huge logistical challenge of actually inoculating most of the world's population
Lockdowns could make this process slower. If fewer people are infected, it will take longer to know whether a vaccine actually works.
The idea of giving people the vaccine and then deliberately infecting them (known as a challenge study) would give quicker answers, but is seen as too dangerous while there is no known treatment.
How many people need to be vaccinated?
It is hard to know without knowing how effective the vaccine is going to be.
It is thought that 60-70% of people needed to be immune to the virus in order to stop it spreading easily (known as herd immunity).
But that would be billions of people around the world if the vaccine worked perfectly.
How do you create a vaccine?
Vaccines harmlessly show viruses or bacteria (or even small parts of them) to the immune system. The body's defences recognise them as an invader and learn how to fight them.
Then if the body is ever exposed for real, it already knows what to do.
The main method of vaccination for decades has been to use the original virus.
The measles, mumps and rubella (MMR) vaccine is made by using weakened viruses that cannot cause a full-blown infection. The seasonal flu jab takes the main strains of flu doing the rounds and completely disables them.
The work on a new coronavirus vaccine is using newer, and less tested, approaches called "plug and play" vaccines. Because we know the genetic code of the new coronavirus, Sars-CoV-2, we have the complete blueprint for building it.
Researchers in Oxford have put small sections of its genetic code into a harmless virus that infects chimpanzees. They hope they have developed a safe virus that looks enough like the coronavirus to produce an immune response.
Other groups are using pieces of raw genetic code (either DNA or RNA depending on the approach) which, once injected into the body, should start producing bits of viral proteins which the immune system again can learn to fight.
Would a vaccine protect people of all ages?
It will, almost inevitably, be less successful in older people, because aged immune systems do not respond as well to immunisation. We see this with the annual flu jab.
It may be possible to overcome this by either giving multiple doses or giving it alongside a chemical (called an adjuvant) that gives the immune system a boost.
Who would get a vaccine?
If a vaccine is developed, then there will be a limited supply, at least initially, so it will be important to prioritise.
Healthcare workers who come into contact with Covid-19 patients would top the list. The disease is most deadly in older people so they would be a priority if the vaccine was effective in this age group.
However, it might be better to vaccinate those who live with or care for the elderly instead.
Читайте также: